Researchers at the University of California, San Francisco (UCSF) and the Gladstone Institutes have uncovered a potential new approach to treating Alzheimer’s disease using two existing FDA-approved cancer drugs. The drugs, letrozole and irinotecan, have been found to reduce tau protein clumps in mice, a key feature of Alzheimer’s, and improve cognitive functions like memory and learning.
Repurposing Cancer Drugs for Alzheimer’s
Both letrozole, which is used to treat breast cancer, and irinotecan, prescribed for colon and lung cancers, are already familiar in the medical world. However, their potential to address Alzheimer’s-related brain damage was not known until recently. By targeting tau proteins, these drugs show promise in reversing some of the damage caused by Alzheimer’s, offering a new avenue for treatment without the need for completely new drug development.
The key to their effectiveness seems to lie in the combination of the two drugs, each targeting different types of brain cells. Letrozole appears to help neurons, the cells responsible for transmitting information, while irinotecan works on glial cells, which support neurons. This dual-target approach could address Alzheimer’s from multiple angles, which is often needed for such a complex disease.
How the Drugs Work
One of the main characteristics of Alzheimer’s is the buildup of tau proteins, which form tangles inside the brain and disrupt normal cell function. This accumulation is a significant factor in the cognitive decline that defines the disease. The combination of letrozole and irinotecan seems to reduce these tau protein clumps in the brain of mice models.
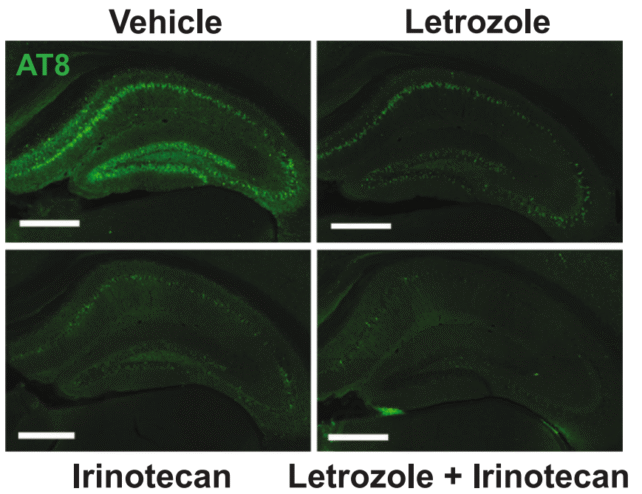
Beyond just clearing tau clumps, the mice treated with the drugs also demonstrated improvements in learning and memory tasks, which are often severely impaired in Alzheimer’s patients. This suggests that letrozole and irinotecan may not only help reduce the physical markers of the disease but could also restore some of the cognitive abilities that are lost.
Moving from Mice to Humans
While the results in mice are promising, the real test lies in whether these drugs can have the same effect on humans. The drugs have already been tested and approved for other types of cancer, so they would face a quicker pathway to clinical trials for Alzheimer’s than entirely new medications. However, before they can be used to treat Alzheimer’s, researchers must first evaluate their potential side effects in this new context.
Still, the fact that these drugs are already FDA-approved could speed up the process. If successful, they could be repurposed for Alzheimer’s treatment, which might provide a much-needed solution for the millions affected by the disease.
A New Approach to Alzheimer’s Treatment
Alzheimer’s disease currently affects over 55 million people worldwide, and that number is expected to rise as the global population ages. The current lack of effective treatments for the disease makes any potential breakthrough significant. If these drugs are shown to work in humans, it could change how Alzheimer’s is treated, offering a new therapeutic strategy for a condition that has long been difficult to address.
In the coming years, further research and clinical trials will be essential to determine whether letrozole and irinotecan can live up to their potential. However, this discovery marks an exciting step toward finding new ways to treat Alzheimer’s and highlights the possibility of repurposing existing drugs for other complex diseases.
The research has been published in Cell.